News & Events
- Filter by
- Categories
- Tags
- Authors
- Show all
- All
- ""Nkcf
- "Augmented reality"
- "Dan Cohen
- "Mojo Vision"
- @brookemesser
- @hajiSaeed @keratoconus @NKCF
- @vancethompsonvision
- 2020
- 2021
- adolescents
- africa
- aiyanna beltran
- allergies
- allergy season
- allergy symptoms
- alphaeon
- ambassador
- ambassadors
- american academy of optometry
- anxiety
- aoa 2017
- ARCH
- artificial tears
- athens
- Athens Protocol
- avedro
- avellino
- avoid
- awareness
- back to school
- bala ambati
- blue cross
- blue shield
- blue shield blue cross
- boston prose
- brian chou
- cairs
- caption contest
- care credit
- care first blue cross
- carl domke
- case
- cause of kc
- children and keratoconus
- cleaning
- Colorado
- common
- complications
- computer eyes
- computer screen
- conjunctivitis
- contact lens
- contact lens care
- contact lens case
- contact lens fitters
- contact lens safety
- contact lens study
- contact lens tips
- contact lenses
- contacts
- contacts for keratoconus
- contest
- cornea
- cornea transplant
- corneal
- corneal collagen crosslinking
- corneal crosslinking
- corneal insert
- corneal lifting
- corneal surgery
- corneal transplant
- corneal transplants
- corona virus
- coverage
- covid
- covid-19
- credit
- crosslinking
- crosslinking coverage
- cxl
- cxl coverage
- cxl covrage
- cxl insurance
- cxl over time
- DALK
- denise scott
- depression
- digital strain
- diseased cornea
- dna
- do's and don'ts
- docs
- doctors
- don't rub your eyes
- down syndrome
- downsyndrome
- Dr S Barry Eiden
- Dr. Emily McCourt
- dr. laurence sperber
- dr. rebekah montes
- dry
- dry and keratoconus
- dry eye
- dry eye causes
- dry eye shop
- dry eye symptoms
- ds
- Dylan muyambo
- e update
- ectatic cornea
- epi off
- epi on
- event
- extensions
- eye
- eye banks
- eye drops
- eye exam
- eye exams
- eye fatigue
- eye infection
- eye injury
- eye protection
- eye rubbing
- eye safety
- eye strain
- eye tests
- eyelash
- families
- FAQ
- february
- feedback
- film festival
- fireworks
- fitting
- frequently asked questions
- ftting
- gabby chaves
- gas permeable
- gene
- genetics
- German
- giving tuesday
- glaucoma test
- goggles
- greece
- hay fever
- healthcare
- High altitude disease
- HOA
- holiday
- how to stop keratoconus
- humans of keratoconus
- hydrops
- hygiene
- indy 500
- infection
- injury
- insurance
- insurance appeal
- insurance reimbursement
- insurance update
- insurange
- INTACS
- intrastromal corneal ring segments
- itchy
- iveena
- jack parker
- jim herschenbach
- joey gase
- johns hopkins
- joseph ciolino
- K-readings
- kaiser
- Kanellopoulos
- Kara Williams
- kc
- kc adolescence
- kc advice
- kc and allergies
- kc booklet
- kc community
- kc coverage
- kc doctor
- kc doctors
- kc families
- kc guide
- kc insurance
- kc research
- kc specialists
- Keratitis
- keratoconus
- keratoconus allergies
- keratoconus ambassador
- keratoconus and allergies
- keratoconus and environment
- keratoconus and military
- keratoconus booklet
- keratoconus doctor
- keratoconus families
- keratoconus insurance
- keratoconus living
- keratoconus numbers
- keratoconus progression
- Keratoconus Seminar
- keratoconus specialists
- keratoconus symposium
- keratoconus treatment
- keratoplasty
- kids
- lens care
- lenses
- link
- living with kc
- made
- marjan farid
- mask
- medica health plans
- mental health
- mental wellness
- midday fogging
- military
- mistakes
- money
- nasal spray
- NASCAR
- national alliance for eye research
- national doctors 2018
- national doctors month
- national sunglasses day
- natural
- news
- newsletter
- nkcf ambassador
- nkcf family symposium
- nkcf roundtable
- nutrifill
- oculus. tomography
- ophthalmologist
- Ophthalmologists
- ophthalmology times
- optometrist
- optometrists
- optometry times
- painful eyes
- pandemic
- patient experience
- patient guide
- patient profile
- patient profiles
- patient referral
- patient resources
- patient stories
- patient story
- patient symposium
- patients of kc
- paying for care
- pediatric
- Penetrating Keratoplasty
- pentacam
- personal stories
- peter hersh
- photo contest
- photography
- photrexa
- piggyback lenses
- piggybacks
- pinguecula
- PK
- poppy mccabe
- position
- pregnancy
- prevalence
- pterygium
- quality of life
- reemergence of kc
- referral list
- referrals
- regents blue cross
- reimbursement
- research
- resources
- RGPlenses
- rhinitis
- runny nose
- safety
- sam guttman
- Save Sight Registry
- scleral fitting
- scleral lens
- scleral lens study
- scleral lenses
- sclerals
- self care
- september
- side effects
- sleep
- sleeping
- soft lenses
- stephanie woo
- stories
- strain
- study
- summer
- sun protection
- sunglasses
- sunlight
- support nkcf
- surgery
- survey
- surveys
- symposium
- teary eyes
- teens
- tips
- to
- tommy pham
- top
- top docs
- top doctors
- transplant
- treatment
- tyrvaya
- unilateral
- united healthcare
- update
- uv rays
- vaccine
- video
- vision test
- water
- work accommodations
- work and kc
- world kc day
April 10, 2024
Originally published in NKCF Update (February 2024) A few years ago, Update presented results of research conducted […]
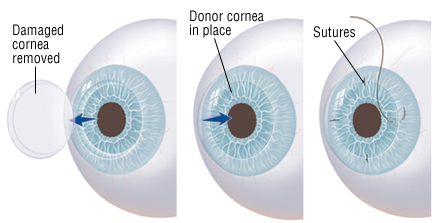
April 3, 2024
Originally published in NKCF Update (February 2024). One mistaken belief by many with keratoconus is that […]
March 25, 2024
Originally published in NKCF Update (February 2024). Individuals born with Down syndrome (DS) face a multitude […]
March 14, 2024
Originally published in NKCF Update (February 2024) An HOA isn’t just a Homeowner’s Association When you […]